” The best way to predict the future is to create it “
(Peter Drucker – Professor, author & theoretician)

At macopharma, we believe in innovation to reach our vision to raise the standards of care. From incremental to radical and disruptive we organize with multi-disciplinary experts: our innovation committee.

What is an innovation?
A new or modified entity that performs or redistributes value (AFNOR, ISO 56000)
The degree of novelty can be expressed by the attributes of an innovation (ISO 56000) :
Incremental innovation : progressive changes (ISO 56000)
Gradual, continuous improvements on existing products, services, processes, …
Radical innovation: entirely new or significant changes (ISO 56000)
is at the other end of the value chain compared to incremental innovation
Ex: technological breakthrough that transform industries – can create new market
Disruptive innovation: replaces established offers (ISO 56000)
As one of the main actors in the promotion of platelet concentrates preparation, our mission is to understand customers’ needs and provide solutions to help them produce the most qualitative and safe products. Our observation on the platelets’ pooling process led us to the conclusion that platelet concentrates preparation is currently time-consuming, demanding and complex. In order to complete its offer and optimize platelet preparation process, Macopharma has therefore designed Maconnect, the very first multiple sterile connection device

Its one-step procedure
allows up to
connections
The time needed to connect
a pool of Buffy Coat is less than
seconds
Especially designed for pooling operations
Maconnect is a unique and innovative concept, especially designed to optimize, simplify and step up the platelets preparation process, while guaranteeing the preparation of a safe platelet concentrate.
Maconnect is the very first multiple sterile connection device, especially designed for pooling operations.
The MacoSeal Light is the new innovation by Macopharma, a cordless sealer with an extended battery autonomy, a unique compact lightweight shape and an outstanding intuitive sealing button enabling every usage configuration.
The remarkable design of the MacoSeal Light was meant to increase the device capabilities while optimising its ergonomics and reducing manual efforts.
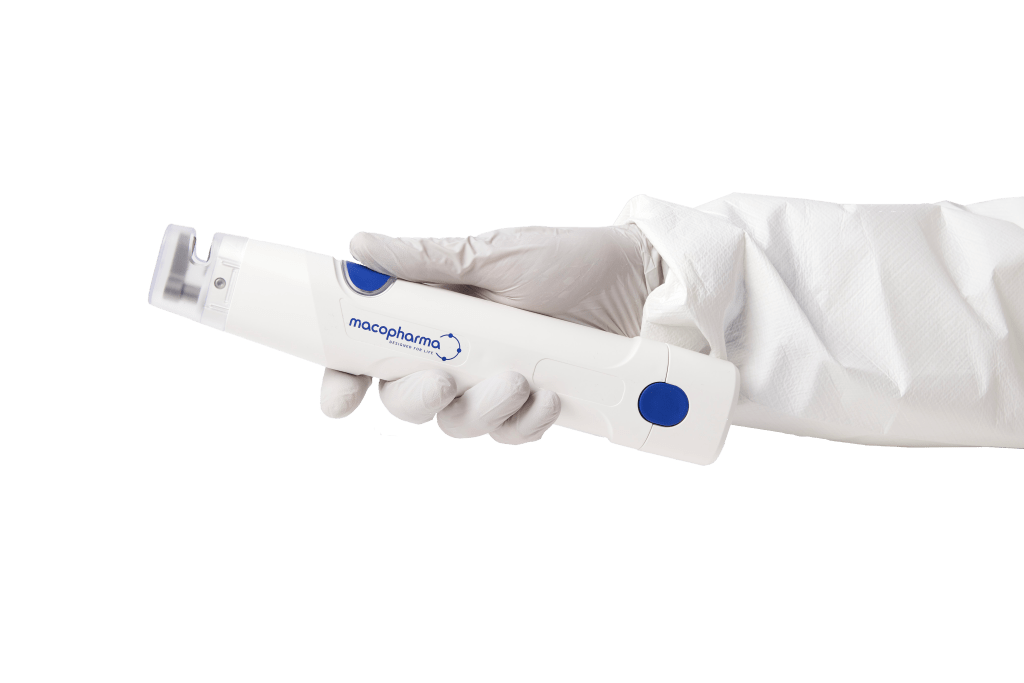
Weight
Complete battery charge
Sealings per battery charge


No compromise:
Macopharma bans DEHP, to ensure SAFE and SECURED
blood component solutions to every patient in the world!
More than words, we commit to act and respect our mission:
We support life !
we consider Non DEHP
transition as INNOVATION.
Because the value of future bag systems is about safety
we have dedicated a full team of scientific experts to research,
design and develop our new kits as a pure
INNOVATION.
Less leaching than DEHP5-7
Alleviate environmental hazard risks
(1) Wirnitzer U, Rickenbacher U, Katerkamp A, Schachtrupp A. Systemic toxicity of di-2-ethylhexyl terephthalate (DEHT) in rodents following four weeks of intravenous exposure. Toxicology Letters 2011; 205 (1): 8-14.
(2) Carlson KR. U.S. Consumer Product Safety Commission (CPSC). Toxicity Review for Di-2-ethylhexyl Terephthalate (DEHT). Risk Science Center Department of Environmental Health, University of Cincinnati, 2018.
(3) SCENIHR (Scientific Committee on Emerging and Newly-Identified Health Risks), Scientific Opinion on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk. 2015.
(4) Kambia N, Séverin I, Farce A, Dahbi L, Dine T, Moreau E, Sautou V, Chagnon MC. Comparative Effects of Di-(2-ethylhexyl)phthalate andDi-(2-ethylhexyl) terephthalate Metabolites on Thyroid Receptors: In Vitro and In Silico Studies. Metabolites 2021; 11:94.
(5) Graminske S, Puca K, Schmidt A, Brooks S, Boerner A, Heldke S, de Arruda Indig M, Brucks M, Kossor D. In vitro evaluation of di(2-ethylhexyl)terephthalate-plasticized polyvinyl chloride blood bags for red blood cell storage in AS-1 and PAGGSM additive solutions. Transfusion 2018; 58:1100-1107
(6) Nielsen BS, Andersen DN, Giovalle E, Bjergstrøm M, Larsen PB. Alternatives to classified phthalates in medical devices. Dan. Minist. Environ. 2014.
(7) Thelliez et al. Migration of di(2-ethylhexyl) phthalate, diisononylcyclohexane-1,2-dicarboxylate and di(2-ethylhexyl) terephthalate from transfusion medical devices in labile blood products: A comparative study. Vox Sang 2023; 118(7):533-542.
* Phosphate-Adenine-Glucose-Guanosine-Saline-Mannitol
** Saline-Adenine-Glucose-Mannitol
Incorporation of PAGGSM in DEHT-based RBC storage bags significantly attenuates haemolysis and enhances key red blood cell quality indicators.1,2
PAGGSM has been used for decades in Germany and enabled to increase the storage time from 42 to 49 days3,4
No impact on clinical effectiveness and patient’s safety reported (Germany, Switzerland)
(1) Larsson L, Sandgren P, Ohlsson S, Derving J, Friis-Christensen T, Daggert F, Frizi N, Reichenberg S, Chatellier S, Diedrich B, Antovic JP, Larsson S, Uhlin M. Non-phthalate plasticizer DEHT preserves adequate blood component quality during storage in PVC blood bags. Vox Sang 2021; 116:60–70.
(2) Graminske S, Puca K, Schmidt A, Brooks S, Boerner A, Heldke S, de Arruda Indig M, Brucks M, Kossor D. In vitro evaluation of di(2-ethylhexyl)terephthalate-plasticized polyvinyl chloride blood bags for red blood cell storage in AS-1 and PAGGSM additive solutions. Transfusion 2018; 58:1100-1107
(3) Hess J. Extended Liquid Storage of Red Blood Cells. In: Blood Donors and the Supply of Blood and Blood Products. Manning FJ, Sparacino L, Eds. Washington DC: The National Academies Press, 1996.
(4) Flegel WA, Natanson C, Klein HG. Does prolonged storage of red blood cells cause harm? Br J Haematol 2014; 165:3-16.
EFS/SFS study
(1) Wirnitzer U, Rickenbacher U, Katerkamp A, Schachtrupp A. Systemic toxicity of di-2-ethylhexyl terephthalate (DEHT) in rodents following four weeks of intravenous exposure. Toxicology Letters 2011; 205 (1): 8-14.
(2) Carlson KR. U.S. Consumer Product Safety Commission (CPSC). Toxicity Review for Di-2-ethylhexyl Terephthalate (DEHT). Risk Science Center Department of Environmental Health, University of Cincinnati, 2018.
(3) SCENIHR (Scientific Committee on Emerging and Newly-Identified Health Risks), Scientific Opinion on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk. 2015.
(4) Kambia N, Séverin I, Farce A, Dahbi L, Dine T, Moreau E, Sautou V, Chagnon MC. Comparative Effects of Di-(2-ethylhexyl)phthalate andDi-(2-ethylhexyl) terephthalate Metabolites on Thyroid Receptors: In Vitro and In Silico Studies. Metabolites 2021; 11:94.
CBS/NETCAD study
Whole blood-derived red blood cell concentrates, collected and stored in prototype DEHT/PAGGSM collection sets, show acceptable quality until expiry at 42 days
Overall, in vitro quality of “warm” RCCs in DEHT/PAGGSM indicated:
Deformability changes observed, likely related to the absence of DEHP at the RBC membrane.
Walsh GM, Howell A, Stephenson T, Olafson C, Blake J, Sumian C, Reichenberg S, Brebant Q, McTaggart K. AABB 2024, P-BC-38.


performed with