Authors
A. Lotens¹, T. Najdovski¹, N. Valensart¹, N. Cellier¹, C. Sumian², Q. Brebant², C. Naegelen³,C. Cretenet³, N.Marpaux³
¹Service du Sang, Belgian Red Cross, Namur, Belgium (Croix-Rouge de Belgique – Ensemble, luttons contre les vulnérabilités)
²MacoPharma, Tourcoing, France
³EFS Bourgogne Franche Comté, Besançon, France (L’EFS en Bourgogne-Franche-Comté | Établissement français du sang)
Introduction and Aim :
→The plasticizer di(2-ethylhexyl)-phthalate (DEHP) is a common component of blood bags.
→Exposure to DEHP is raising concern on about its potential carcinogenicity and reprotoxicity.
→DEHP will be banned following the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)
regulation in 2030.
→AIM:Assess the impact of different process preparations on the RBC quality stored in di(2-ethylhexyl)-terephthalate DEHT (Transfufol DEHT 3126 from RENOLIT Nederland B.V.)/PAGGSM (Phosphate Adenine Glucose Guanosine Saline Mannitol)
Methods :
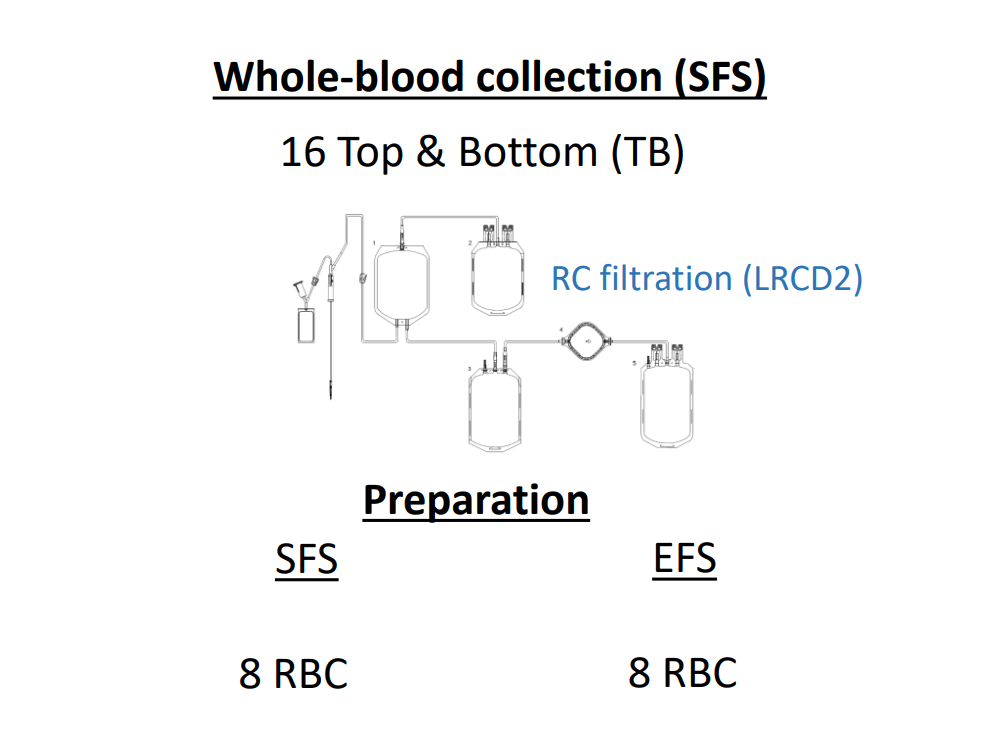
Results :
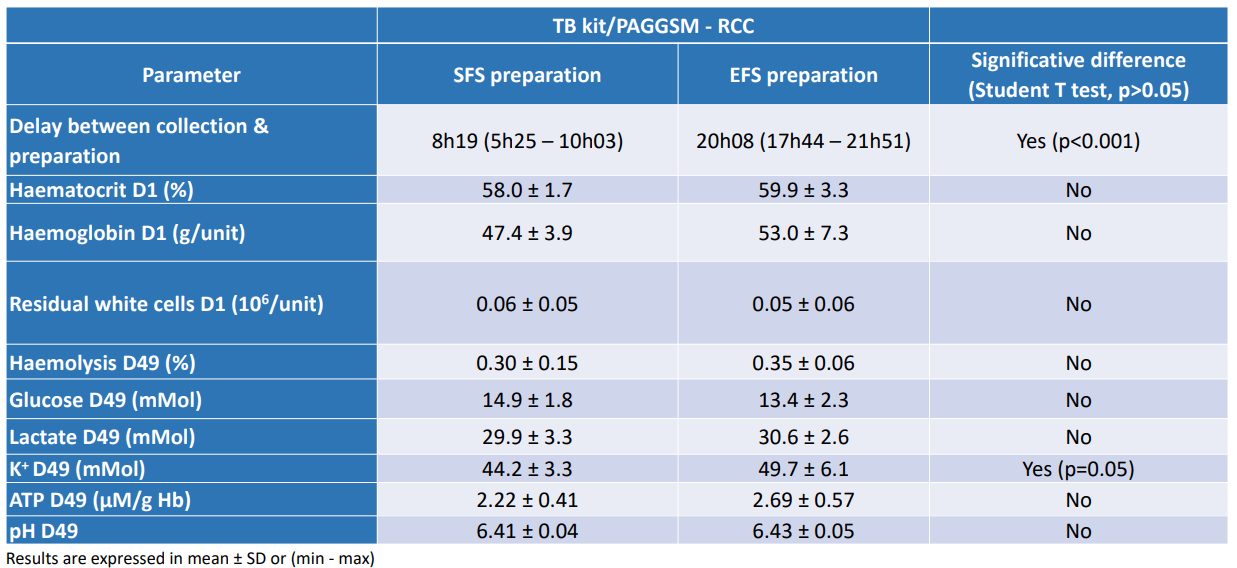
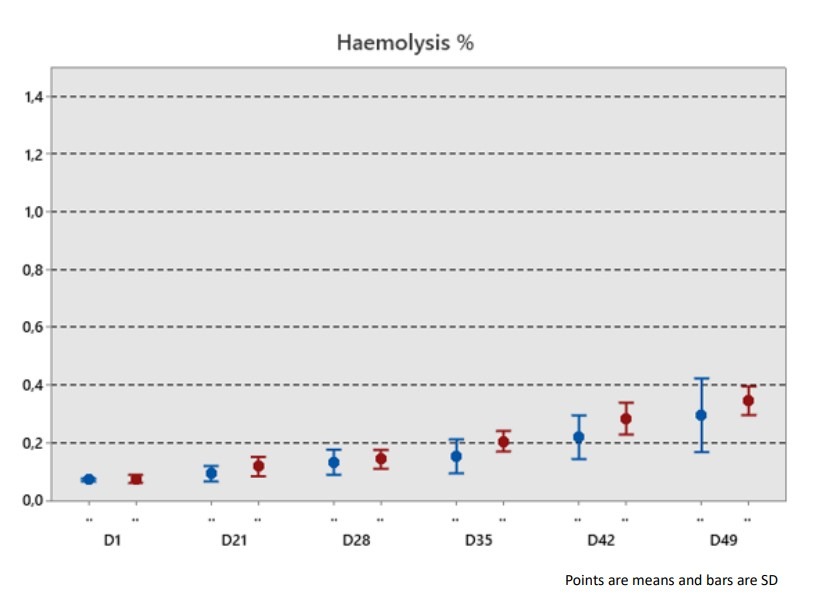
Conclusion :
There is no significant impact on the different process preparations on the RCC quality with a combination of DEHT-PAGGSM, outside of the potassium that is more pronounced when the process is performed above 12 hours after WB collection.
Lear more about our DEHT/PAGGSM solution : Discover Innovations – At Macopharma, we believe in innovation !